PV Working Principle
Principle of solar energy: The Photovoltaic effect
Photovoltaic (PV) effect is the conversion of sunlight energy into electricity.
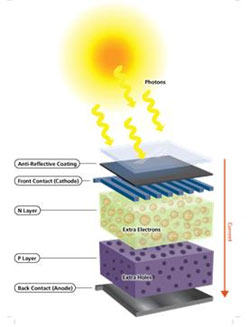
In a PV system, the PV cells exercise this effect. Semi-conducting materials in the PV cell are doped to form P-N structure as an internal electric field. The p-type (positive) silicon has the tendency to give up electrons and acquire holes while the n-type (negative) silicon accepts electrons. When sunlight hit the cell, the photons in light excite some of the electrons in the semiconductors to become electron-hole (negative-positive) pairs. Since there is an internal electric field, these pairs are induced to separate. As a consequence, the electrons move to the negative electrode while the holes move to the positive electrode. A conducting wire connects the negative electrode, the load, and the positive electrode in series to form a circuit. As a result, an electric current is generated to supply the external load. This is how PV effect works in a solar cell.
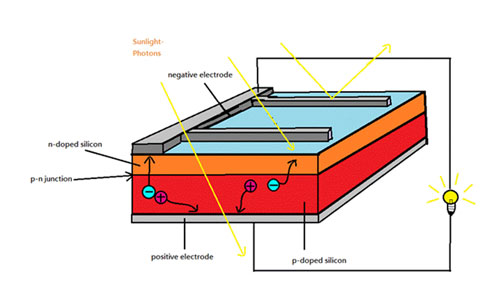
How does PV Cells Work
p-type and n-type materials
Solar cells are running on junction effect principle. To understand junction effect, we should understand n-type and p-type material. Doping process is needed to obtain n-type or p-type material. Doping means inserting another atom into the bulk crystal. Consider silicon crystal: each silicon atom has four electrons in its valance band and these electrons make bonds with other Silicon atom. You can see the silicon crystal in the left side with valance electrons of each Si atom. Note that we call that structure as crystal since all Si atoms are perfectly aligned. We can convert this structure in to n-type or p-type by doping different atoms. For example let’s dope it by boron. Boron atom has 3 electrons in its valance band. When we insert B atom instead of a Si atom, one bond between B atom and a Si atom will be very weak. To complete the prefect symettry in this structer, crystal willbe aimed to catch an external electron. As you can see an electron is missing since B atom has 3 electron in its valence band. This missing bond can be treated a positively charged particle called ‘hole’. This material is called p-type material. What if we dope Phosphorous atom instead of Boron atom? Phosphorous atom has 5 electrons in its valance band.
When P atom is inserted into the Si lattice, 4 electrons will be able make bond with neighbour Si atoms. However 5th electron will be hanged on. So, it will be in an energy level that very close to conduction band since it will be nearly free. This nearly free electron can easily leave P atom with a small thermal energy. Note that there is an extra electron in this new structure. So we call this new material n-type material. In contrast to p-type material, n-type material has a tendency to give electrons. Consequently we have two types of materials. One wants to give electrons and the other wants to receive electrons. We can create a p-n junction by bringing them together.
p-n junction
When we bring p-type and n-type material together, a diffusion occurs on the surface between them. Electrons starts to diffuse from n-type to p-type. Similarly, holes diffuses from p-type region to n-type region. This diffusion creates aelectron-hole free region in a very short distance at the interface region. This thin layer is called depletion region.
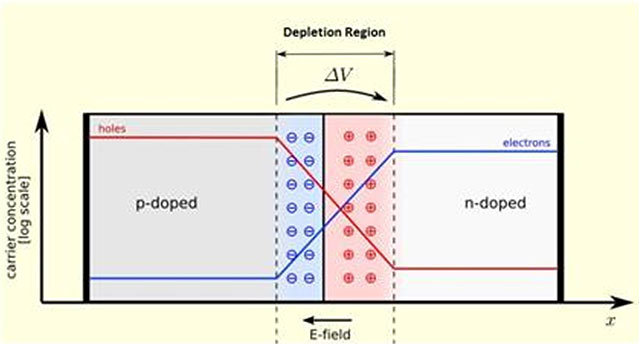
can see the diffusion in deplete on in figure. Blue line indicates the electron concentration while red line indicate whole concentration trough semiconductor material. As you see there is an electric field from the n-side to the p-side of the depletion region. Since the electrons are negative charges this electric field applies a force to an electron entering the depletion region. Any electron generated by sun light in the vicinity of the depletion region may pass to the n-side of the junction very easily. If we connect a wire or any load between the ends of n-type and p-type region with metal contacts, this electron will flow to the p-type through this external load. So we need an external energy to create this current: something should energize the electrons in the p-type region to enter depletion region. Solar radiation is an excellent energy source to do this job. The solar cell type explained above is the example of first generation, wafer based Crystal Silicon solar cells. There are some differences in the structure of other solar cell types. But the basic principle is the same: some kind of p-n junction (or similar potential energy profile) has to be used to convert the solar radiation to electrical energy. Different photovoltaic cell types are discussed in “What are the PV cell types?” page.